
The municipal wastewater is routinely applied for soil irrigation in peri-urban agriculture of the country.

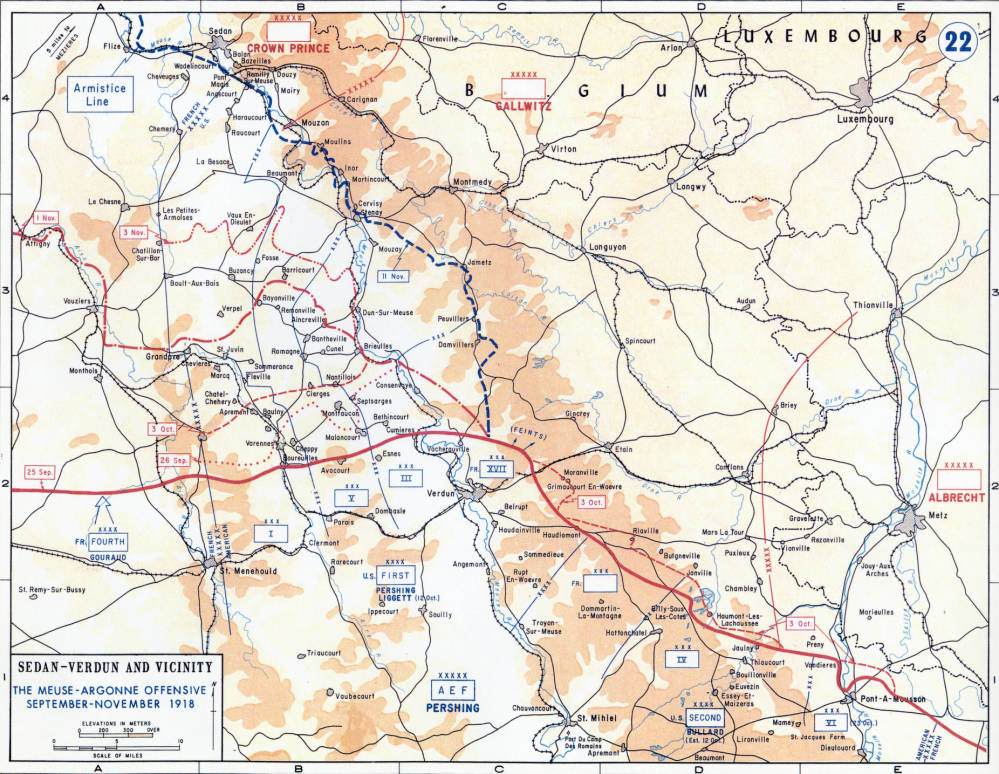
The reducing environment of the aquifer system becomes oxic at the shallow depth due to comparatively shallow groundwater level, and impressive water level fluctuation resulting in vertical mixing of anthropogenic As contaminants.ĭespite considerable research of arsenic (As) level in ground/drinking water of Pakistan, scarce data is available regarding irrigation water contamination by As and associated health risks. Further, it indicates the dominance of carbonate weathering and relatively high pH values (range: 8.00-9.00) helps to release As in groundwater. Groundwater is alkaline with a high concentration of HCO3- as compared to other chemical parameters. The results show that the highest As concentration, which is 8-times more than the permissible limit, is observed at Gyantoli village in Begusarai district in Bihar state. The anthropogenic addition of As in groundwater was separated by the estimated inflection point. The natural background level is a crucial parameter for identifying and quantifying groundwater pollution and assessing measures to control pollution. The natural background level of As was also estimated using Grubb’s test and cumulative probability plots. Hydrogeochemical analytical data related to the As concentration had been analyzed to identify the As sources in groundwater of the active floodplains of the Ganga basin in Northern India. As problem of groundwater in Bhagirathi–Ganga deltaic plain is well-known for over the last three decades. Recently around 40 million inhabitants of the world are living in the hazardous zone having groundwater As level >50 µg/L. Risk management measures have been successful both in Freiberg and in Verdun.Īrsenic contaminated groundwater is seen as one of the most critical routes of human exposure to geogenic pollutants. We recommend the risk management procedure developed in Germany for other parts of the world where both geogenic and anthropogenic arsenic is present in agricultural soil and water. Risk management is the main option for extensive areas, while remediation options that directly treat the soil can only be considered in small areas subject to sensitive use. The main results included: (1) pot experiments using a biologically synthesized adsorbent (sorpP) with spring barley reduced the mobility of arsenic, (2) the Omega-3 Index ecotoxicological tests verified that sorpP reduced the uptake and toxicity of arsenic in plants, (3) reverse osmosis membrane systems provided 99.5% removal efficiency of arsenic from surface water, (4) the sustainability assessment revealed that adsorption and coagulation–filtration processes were the most feasible options for the treatment of surface waters with significant arsenic concentrations, and (5) a model was developed for assessing health risk due to arsenic exposure. We investigated the behaviour, remediation and risk management of arsenic in Freiberg, Germany, characterized by past mining activities, and near Verdun in France, where World War I ammunition was destroyed. We assume that this effect is of high relevance especially for reservoirs impounding small streams with forested catchments and weakly acid buffering parent material of soil formation.Ĭhronic exposure to arsenic may be detrimental to health. Leaching from the catchment soils partially enriches elements in stream sediments before their fine-grained portions in particular are deposited as reservoir sediment. This is enabled by a diffuse element release from the soils into the impounded streams, which is particularly favored by soil acidity. The enrichments of cadmium (EF: 8.2), manganese (EF: 3.9), nickel (EF: 4.8), and zinc (EF: 5.0) are significantly higher.
The median values of the element-specific enrichment factor (EF) demonstrates slight enrichments of arsenic (EF: 3.4), chromium (EF: 2.8), and vanadium (EF: 2.9) for reservoir sediments.

The results indicate that reservoirs generally have a high potential for contaminated sediment accumulation due to preferential deposition of fine particles. To address this issue, the concentrations and the mobile fractions of metal(loid)s were determined in the sediments and the respective catchment areas of six reservoirs. Knowledge concerning the sources of potential contaminants is therefore of important significance. Sediment management is a fundamental part of reservoir operation, but it is often complicated by metal(loid) enrichment in sediments.


 0 kommentar(er)
0 kommentar(er)
